Chapter 0 - The Prologue - Test Review
Instructions: Click on the button to see if your answer is correct or not. I recommend keeping track of how many you get correct on your first try.
The rock is black and shiny.
The rock dates from the Precambrian Era.
The rock cooled very rapidly.
The rock formed deep in the Earth's interior.
Using a ruler to measure the length of a stick is an example of
predicting the length of the stick by guessing.
measuring the rate of change of the stick by making inferences.
calculating the percent of error by using a proportion.
extending the sense of sight by using an instrument.
A group of students observed and measured various characteristics of a stream for one day. Which statement about the stream is most likely an inference?
The velocity of the stream is greatest near the outside of a meander.
The stream's depth is different at various distances from the streambank.
The stream water is dark brown.
The water level of the stream will rise after the next rainfall.
In order to make observations, an observer must always use
proportions.
experiments.
the senses.
mathematical calculations.
a fact.
a measurement.
an inference.
an observation.
A rain gauge records three inches of rain in less than one hour.
Damage from the storm is expected to be extensive.
The windspeed is measured at 200 km/hr.
The central air pressure is recorded at 946.0 mb.
measurement of air temperature.
reading of atmospheric pressure.
determination of dewpoint temperature.
weather forecast for 3 days.
![]()
Use the picture below to answer the question.
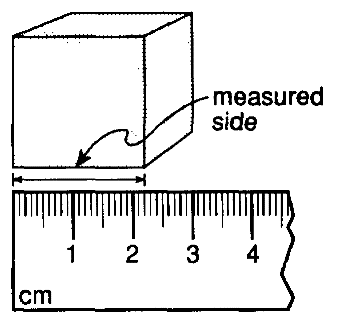
If each side of the cube shown above has the same length as the measured side, what is the approximate volume of the cube?
2.20 cm3
4.84 cm3
6.60 cm3
10.65 cm3
![]()
19.15
9.58
3.25
3.26
1.234 X 1011
12.34 X 10-11
1.234 X 10-11
1234 X 10-10
Which of the following is NOT correct?
4.5 X 100 = 4.5
3.9 X 10-4 = 0.00039
3.21 X 10-5 = 321,000
6.8 X 101 = 68
What is another way to write out 4,500?
4.5 X 103
4.5 X 10-3
4.5 X 102
4.5 X 10-2
If you write out 123.456 X 10-4, you will get
0.0123456
1,234,560
1,234,560,000
0.000123456
Mass can be defined as
the amount of matter in an object.
the amount of space in an object.
the amount of matter in a given amount of space.
the effect of the force of gravity on mass.
Volume can be defined as
the amount of matter in an object.
the amount of space in an object.
the amount of matter in a given amount of space.
the effect of the force of gravity on mass.
Weight can be defined as
the amount of matter in an object.
the amount of space in an object.
the amount of matter in a given amount of space.
the effect of the force of gravity on mass.
Density can be defined as
the amount of matter in an object.
the amount of space in an object.
the amount of matter in a given amount of space.
the effect of the force of gravity on mass.
![]()
The graph below shows the relationship between the mass and volume of a mineral.
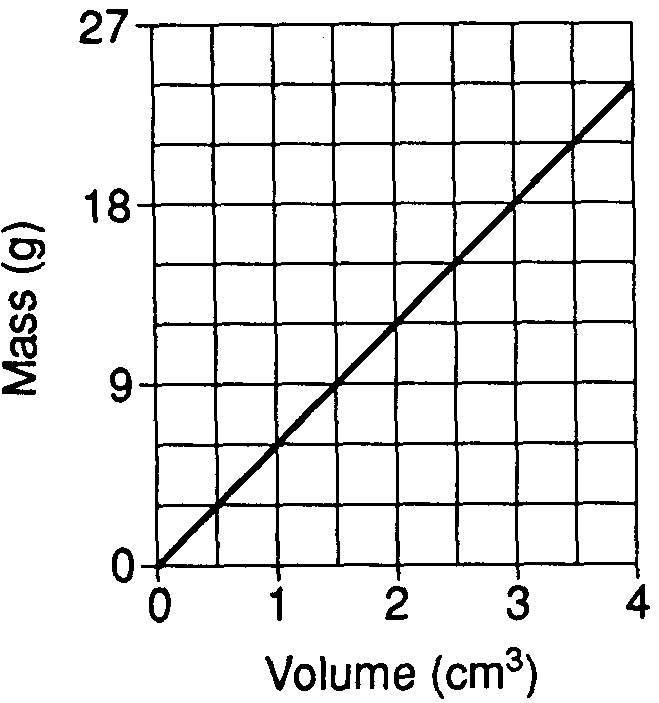
What is the density of this mineral?
3.0 g/cm3
4.5 g/cm3
6.0 g/cm3
9.0 g/cm3
![]()
Base your answers to questions 19 through 21 on the picture below. The mass of the empty 1,000 mL graduated cylinder is 250.0 grams. When filled with the gray liquid (shown below), the graduated cylinder has a mass of 1,300 grams.
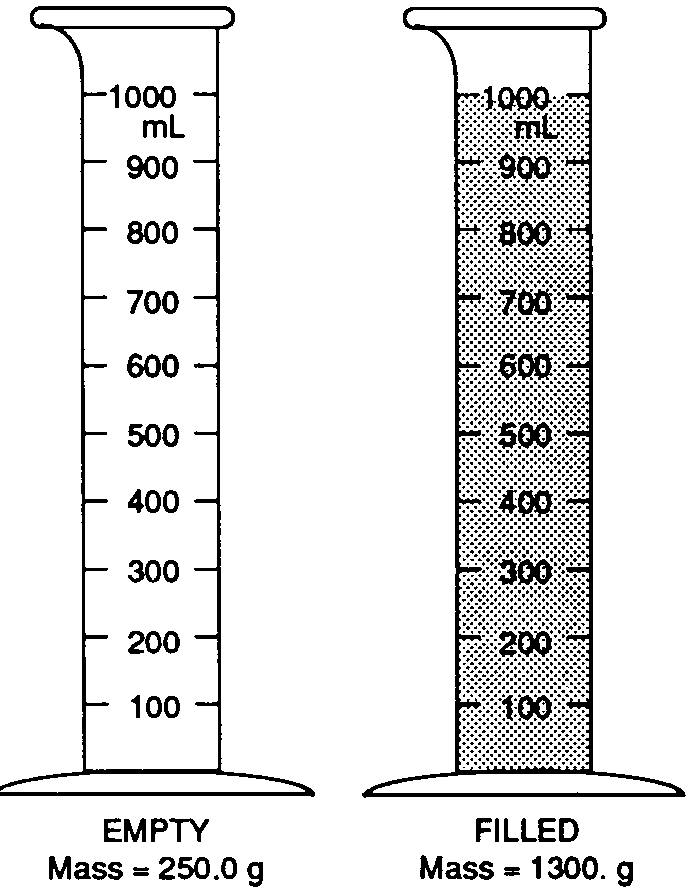
What is the density of this liquid?
0.95 g/ml
1.00 g/ml
1.05 g/ml
1.30 g/ml
What would happen if this liquid was poured into a beaker containing 1,000 ml of water, and providing that the two liquids do not mix?
The water will float on top of the other (gray) liquid.
The other (gray) liquid will float on top of the water.
Part of the water will float on the gray liquid while part sinks below it.
Both liquids will be found in equal portions everywhere throughout the container.
What will happen if you drop a cube into both liquids if the cube has the length of 3 cm and has the mass of 27.81 grams?
The cube will float in both liquids.
The cube will sink in both liquids.
The cube will float on the water, but sink in the gray liquid.
The cube will float on the gray liquid, but sink in the water.
![]()
Use the image below to answer questions 22 through 29.
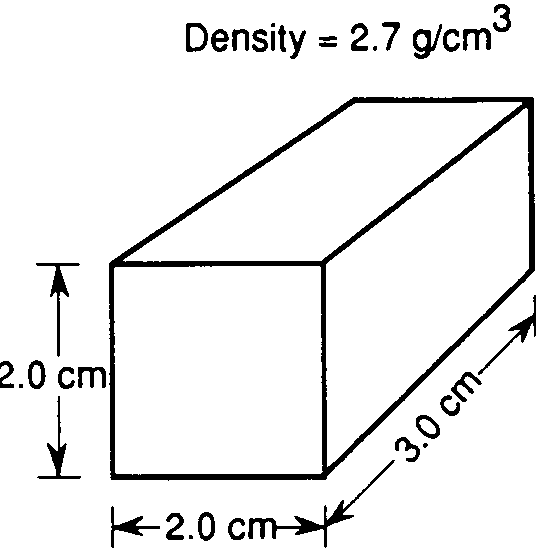
What is the density of this box if the mass is 35 grams?
1.75
0.57
700
8.75
If this block was cut into eight even pieces, what would happen to the density of each of the smaller pieces?
The density would increase.
The density would decrease.
The density would stay the same.
If this block was like most other substances, what would happen to its mass if it is heated?
Its mass would increase.
Its mass would decrease.
Its mass would stay the same.
If this block was like most other substances, what would happen to its volume if it is heated?
Its mass would increase.
Its mass would decrease.
Its mass would stay the same.
If this block was like most other substances, what would happen to its density if it is heated?
Its mass would increase.
Its mass would decrease.
Its mass would stay the same.
If this block was like most other substances, what would happen to its mass if heat energy was removed?
Its mass would increase.
Its mass would decrease.
Its mass would stay the same.
If this block was like most other substances, what would happen to its volume if heat energy was removed?
Its mass would increase.
Its mass would decrease.
Its mass would stay the same.
If this block was like most other substances, what would happen to its density if heat energy was removed?
Its mass would increase.
Its mass would decrease.
Its mass would stay the same.
![]()
Base your answers to questions 30 through 33 on the Earth Science Reference Tables, the diagrams below, and your knowledge of Earth science. The diagrams represent two different solid, uniform materials cut into cubes A and B.
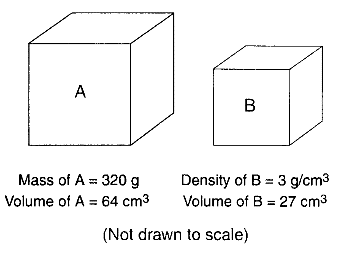
Assume cube B was broken into many irregularly shaped pieces. Compared to the density of the entire cube, the density of one of the pieces would be
greater.
less.
the same.
What is the density of cube A?
0.2 g/cm3
5.0 g/cm3
12.8 g/cm3
64.0 g/cm3
What is the mass of cube B?
3 g
9 g
27 g
81 g
A student calculates the density of a third material as 8.3 grams per cubic centimeter instead of the accepted value of 8.0 grams per cubic centimeter. What is the studentís approximate percent deviation (percent of error)?
3.0%
3.8%
30.0%
36.1%
![]()
Base your answers to questions 34 and 35 on the Earth Science Reference Tables and the diagram below, which represents a solid material of uniform composition.
If this block has a mass of 60 grams and a density of 2.5 g/cm3, what is the length of the side of the box labeled "X"?
24.0 cm3
2.4 cm3
15.0 cm3
The value of "X" cannot be determined.
Which graph best represents the relationship between the mass and volume of various-sized pieces of this material?
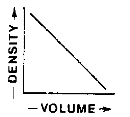

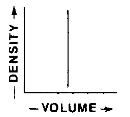
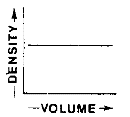
![]()
The cylinder below contains three different liquids. Each liquid has a different density, but will not mix with the other liquids.
What would happen if you were to drop a 1.8 g/cm3 glass bead into this cylinder?
The bead will pass through layers A, B, and C and rest on the bottom.
The bead will sink through layer A and float on layer B.
The bead will sink through layer A, float on layer C, and would be suspended in layer B.
The bead will float on all three layers, but only if each layer was separated from the others.
![]()
Ice floats on liquid water, because, compared to liquid water, the ice
has more mass.
has less mass.
has more density.
has less density.
Which graph most likely illustrates a cyclic change?
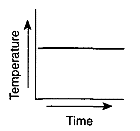
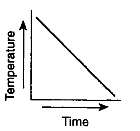
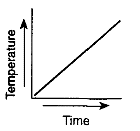

Which graph has a direct relationship between temperature and time?




Which graph has an inverse relationship between temperature and time?




A student finds the mass of a mineral to be 5.5 grams. What is the percent deviation if the textbook says that the mass should 5.0 grams?
1.1%
10.0%
9.1%
0.1%
If the accepted value is 3.2 g/ml and the measured value is 3.9 g/ml, what is the percent deviation?
82.1%
121.9%
17.9%
21.9%
yes buttons